Boosting cubane synthesis with photosensitizers

Leaping to a greener approach for cubane synthesis
Cubane (CH)8 is an organic molecule of intrigue. The journey of cubane synthesis is nothing short of elegance that encompasses the accidental preparation of octaphenylcubane by H H Freedman and D R Peterson, followed by Philip Eaton and Thomas Cole’s seminal synthesis.
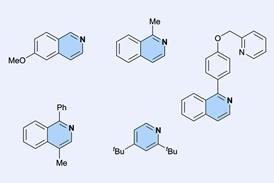
Cubane is a highly strained hydrocarbon with a perfect cubic symmetry, rendering it thermodynamically unstable. Due to high strain energy, cubane displays a large energy barrier for its ring opening and remarkable kinetic stability beyond 200°C. Cubane is a charming contender to benzene as both share close geometries, and are called bioisosteres. If functionalised at a few or all the carbon atoms, they have the prowess to exert pharmacokinetic properties and even replace phenyl (benzene) rings in potential drug candidates. Today, we are obtaining cubyl complexes and even rearranging cubanes to cuneanes and more recently, 1-azahomocubane.