Mystery surrounding metalation reaction’s reagent excess solved

Multiple ‘base-eating’ aggregates discovered in classic directed ortho lithiation
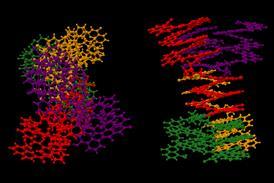
A number of surprisingly complex aggregates have been discovered in the directed ortho metalation, a classic reaction discovered more than 80 years ago. They are the reason this reaction requires a huge excess of butyllithium base: the aggregates ‘cannibalise’ the base and stop it from reacting.
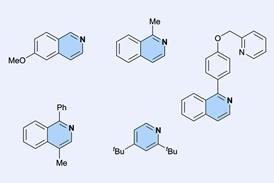
Discovered independently by Georg Wittig and Henry Gilman in 1939, the directed ortho metalation is a type of electrophilic aromatic substitution. In it, an organolithium base such as n-butyllithium (BuLi) deprotonates an aromatic ring. This is directed by a heteroatom-containing substituent on the ring – such as a methoxy or an amide – so the reaction takes place exclusively ortho to this group. The aryllithium intermediate can then react with any number of electrophiles, making this an easy way to create functionalised aromatics.