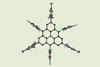
First synthesis of aza-triangulene in solution
Chemists synthesised and isolated unstable species and its cation in crystalline form
For the first time, chemists have synthesised single crystals of aza-triangulene and its cation. These high-spin species are especially unstable – and until now they were only accessible via on-surface synthetic methods, which require complex manipulations with microscopes.
Starting from an amine decorated with aromatic groups, chemists built the structure of aza-triangulene through a series of Suzuki couplings, cyclisations and redox reactions. The addition of bulky electron-withdrawing groups stabilises the structure, extracting energy density from the unpaired electrons and blocking the most reactive sites of the molecule. This strategy – dubbed heteroatom doping – could find further applications in the preparation of other unstable species.




