Bastimolide B
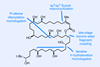
Fewer steps with boron chemistry
Variety – that’s one of the things I love about doing total synthesis. One day you could be using baker’s yeast, the next an air-sensitive molybdenum carbenoid or a retina-searing mercury lamp or a potentiostat. By the time I’d got my PhD I’d run just over 2000 reactions using dozens of metals and catalysts under a dizzying range of reaction conditions. I wasn’t exactly a master at anything (except maybe flash chromatography), but I’d run enough named reactions to fill a book.
In contrast, for all the time we spend marvelling at the beauty of complex natural products, nature actually has very few reactions at her disposal and runs everything under pretty much the same conditions. Although it’s not always obvious at first glance, even the most intricate natural products are built up from simple repeated monomers using just a handful of steps over and over again. For example, a macrolide like bastimolide B is typically assembled via the polyketide synthesis pathway using interactive condensations, decarboxylations, dehydrations and reductions – essentially all undergraduate chemistry – and a just dozen or so shots of the metabolic intermediate acetyl-CoA.




