Uranium’s strong covalent bond breaks periodic table predictions
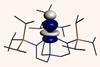
Actinide’s unusual covalency could explain its ability to fix nitrogen
Uranium forms an unusually strong triple covalent bond with nitrile groups, confounding predictions about the bond strength of actinides compared with the remainder of the periodic table.
Elements usually follow a pattern in the strength of their covalent bonds, with groups 1 and 2 forming more ionic bonds and covalency increasing as you move across the periodic table. While the bond strengths of actinides have not been studied in depth, they are likely to sit between the ionic lanthanides and the more covalent d-block elements.




