Close menu
- Home
- News
- Research
- Opinion
- Features
- Culture
- Careers
- Podcasts
- Webinars
-
Collections
- Back to parent navigation item
- Collections
- The future of energy storage
- AI and automation in chemistry
- Sustainable labs
- Research culture
- Coronavirus
- Nobel prize
-
Themed supplements
- Back to parent navigation item
- Themed supplements
- Problem solvers
- Inspiring science
- Up to the challenge
- Eureka moments
- Chemistry 4.0
- Forefront of pharma
- Collaborative chemistry
- Everyday chemistry
- Future of pharma
- Voices in chemistry
- Chemistry detectives
- Innovators
- Green and sustainable chemistry
- Health technology
- View all
- Partner collections
- Register
Chemists reconsider C–H and C–C bond length rationale
By Frances Addison2021-07-08T14:32:00
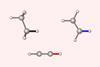
Source: © 2021 Pascal Vermeeren et al
Quantitative proof for steric repulsion theory
Pauli repulsion, rather than orbital interactions, is the driving force behind why C–C and C–H bonds contract as the number of substituents surrounding the carbon centre decreases, new research shows.
- This website collects cookies to deliver a better user experience. See how this site uses cookies.
- This website collects cookies to deliver a better user experience. Do not sell my personal data.
- Este site coleta cookies para oferecer uma melhor experiência ao usuário. Veja como este site usa cookies.
Site powered by Webvision Cloud




